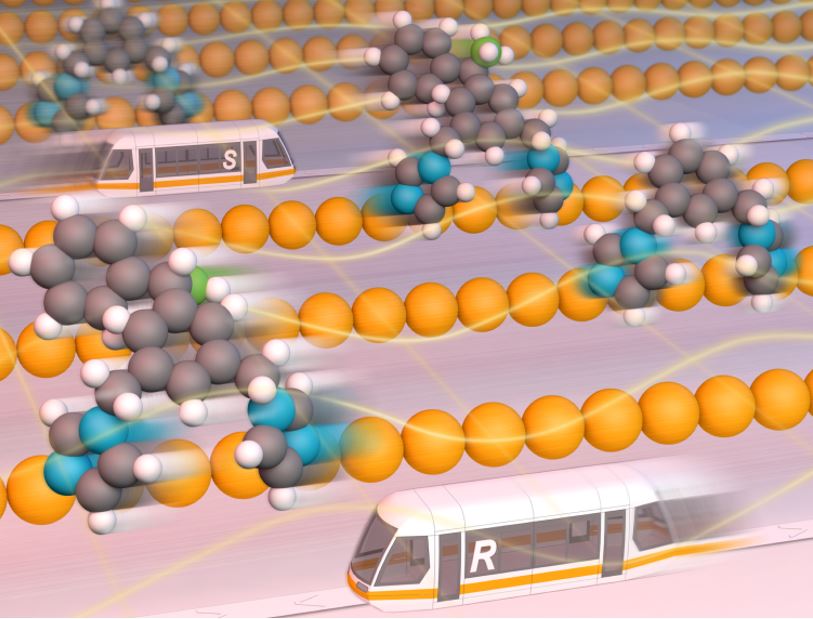
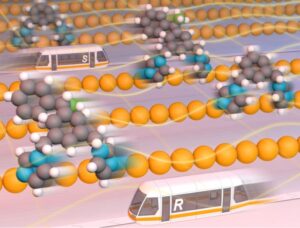
We demonstrate a natural mechanism in which, under certain conditions, one molecular type (e.g. an enantiomer) moves towards one direction, while the other – in the opposite one, separating them. This might be of importance due to e.g. a fact that all living beings are homochiral (composed by only one of the two possible enantiomers), and hence this result may shed some light in answering fundamental questions of the origin of life, as well as of the emergence of homochirality. On a more practical side, this work may stimulate crerating practical methods for seprating different molecules in surfaces, e.g. in racemic mixtures.
On ARCHER and Thomas HEC we have conducted quantum-mechanical simulations of the one-dimensional diffusion of a real chiral molecule (called BIPEB in the following) on a copper surface.This molecule diffuses along a single dimension on top of a row of Cu atoms, as if it were on a train’s track. It stands on the surface with two “legs” formed by its nitrogen atoms binding to Cu atoms underneath, and moves thermally by performing two steps. This is a two-stage mechanism, in which the two legs either are close or away from each other, reminiscent of an inchworm movement. The key is that the energy cost to overcome each step is different, and therefore its diffusion mechanism is asymmetric.
Under equilibrium conditions, the levo and dextro isomers of a racemic mixture of BIPEB molecules move on the surface in either direction indistinctly, following a Brownian motion. In other words, there are equal numbers of molecules moving to the right and to the left; the diffusion is fully random. As levo and dextro are mirror images of each other, their diffusion mechanisms are also related by the same specular symmetry. If we have all the BIPEB molecules equally aligned on a copper surface and apply an external stimulus that forces them to move to the right, they will all move to the right. Applying the field in the reverse direction would move the molecules to the left. Hence, separation is not possible if such a field is applied. Surprisingly, however, given the asymmetry in the diffusion mechanism, one of the enantiomers under the influence of the field “acting to the right” will move faster to the right, while the other enantiomer would move faster to the left in the reversed field, compared to the other enantiomer in each case. Therefore, if we apply a field that oscillates periodically between both directions, each of the two types of enantiomers will move in the direction towards which it moves faster, and hence their separation becomes feasible. We can play with the parameters of this external field, making it more or less “biased” towards one or the other direction, and thus control the separation of the enantiomers even better. Hence, one may be able to obtain net enantiopure accumulations of either levo and dextro-BIPEB in certain regions of the copper surface. A molecular machine (specifically, a ratchet) is thus achieved that could solve racemic mixtures by means of intelligent control of the stochastic motion of the molecules. This method can also be applied to other types of molecular mixtures.
Outcomes:
- New knowledge: a new ab initio based method developed for studying a long-time diffusion of molecules on surfaces
- New technology: we realised how molecules with different potential energy surfaces can be separated on a surface
- New collaborations: a theoretical group in Oviedo University (Prof J. Manuel Recio)
Collaborators:
David Abbasi-Perez – KCL physics; Hongqian Sang – KCL physics and Institute for Interdisciplinary Research, Jianghan University, Wuhan 430056, China; Rasmita Raval – Surface Science Research Centre, Department of Chemistry, University of Liverpool, Liverpool L69 3BX, UK; David Amabilino, Dr Lluisa Perez-Garcia – School of Chemistry, GSK Carbon Neutral Lab. for Sustainable Chemistry, University of Nottingham, Triumph Road, NG7 2TU, UK; J. Manuel Recio – MALTA-Consolider Team and Department of Analytical and Physical Chemistry, Universidad de Oviedo, Oviedo, 33006, Spain; Andrea Floris – School of Chemistry, University of Lincoln, Brayford Pool, Lincoln LN6 7TS, UK
Funding:
- UK EPSRC grant EP/J019844/1 (£325,919) in part by UK EPSRC grant EP/N023587/1 (£358,292)
- National Natural Science Foundation of China (Grant No. 21603086) and the China Scholarship Council (Grant No. 201608420186)
- The Spanish MINECO and FICYT (Projects CTQ2015-67755-C2-2-R, FC-GRUPIN-IDI/2018/000177) and Universidad de Oviedo for a mobility grant to visit King’s College London.
Contact:
Prof Kantorovich, stqa8038@kcl.ac.uk