A new metal-organic framework composed of zinc ions and tripeptide molecules was synthesised in our group and shown to adopt nine distinct crystal structures in a variety of solvents. We exploited this, using mixtures of solvents to control the crystal structure to selectively trigger absorption of guest molecules into the material. Combining experiment and computation, we illustrated the principles of the structural flexibility and activity of this new material in terms of the host potential energy surface and the perturbations caused by the guest-host interactions. This work offers exciting scientific possibilities, for example in catalysis, through the design of materials that can dynamically select the structure needed for a particular task.
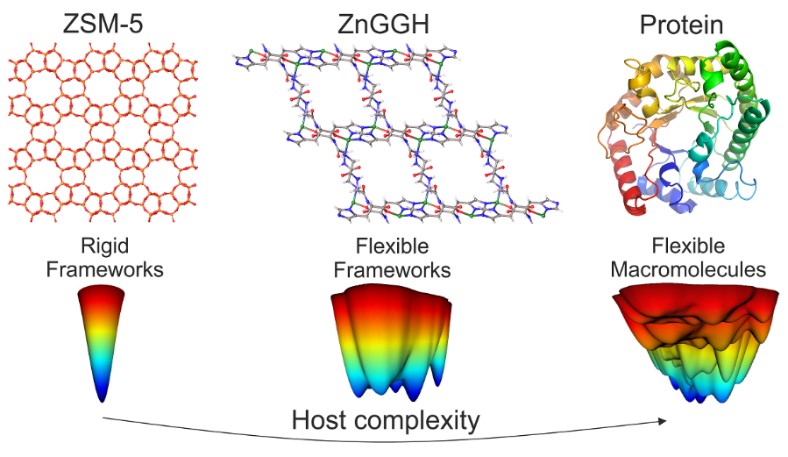
Porous materials such as zeolites are widely used in industry as catalysts for the production of fuels and chemicals and in environmental remediation technologies as adsorbers for the removal of harmful compounds from air and water. These materials are rigid, unlike proteins that can change their structures to carry out chemical processes in response to their environment. We designed a new crystalline synthetic material that is both porous and conformationally flexible (Fig. 1). It is made of Zn2+ ions interconnected by deprotonated glycine-glycine-L-histidine (GGH) tripeptide linkers.
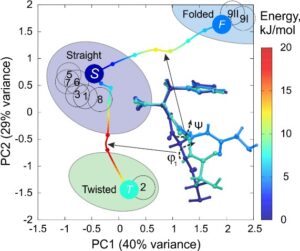
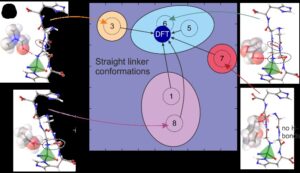
Like a protein, the new porous material ZnGGH can adopt multiple structures, and it can be controllably transformed from one structure to another by changes in its chemical environment – the structure of ZnGGH changes depending on the interactions with guest molecules in its pores. Since different solvents sometimes resulted in similar pore structures, we investigated a library of twelve solvents and identified nine distinct crystal structures including a desolvated structure. To quantify the similarity in linker conformations observed experimentally, we used dihedral angle Principal Component Analysis (dPCA) that revealed the underlying conformational energy landscape computed using DFT (Fig. 2a). Most guests produced structures with a single “straight” linker conformation close to that observed in the global minimum, while DMSO molecules selected a high energy minimum of a “twisted” conformation. The details of guest packing and guest-host interactions resulted in conformational adjustment of the tripeptides of the host (Fig 2b).
Fig. 1. Metal-Organic Framework ZnGGH is both porous and flexible.
A:
B:
Fig. 2. a) Dihedral angle Principal Component Analysis (dPCA) of linker conformation in structures from 1 to 9 (numbered circles) show three clusters of conformations (Straight, Twisted and Folded) that are preferred by the framework. The empty structures predicted by DFT (circles coloured according to their energies) indicate the corresponding local energy minimums that are separated by barriers calculated using Nudged Elastic Band method; b) dPCA for exclusively straight linker conformations shows clustering of structures produced by guest-host interactions that alter the intra-framework hydrogen bond pattern (dashed ellipses).
The DFT calculations performed on Archer allowed us to explore the energy landscape of ZnGGH and accurately identify the role of host-guest interactions in stabilising different minima. We also used DFT simulations to explore alternative peptide sequences to inform future experimental work. Through the design of porous materials that can dynamically alter their pore structure in response to chemical stimuli, this work offers exciting possibilities in areas such as separation and catalysis.
Outcomes:
- The discovery of a new porous flexible material adopting many distinct crystal structures
- A proof of concept of chemically controlling pore shape to trigger uptake of guest molecules
- Paper: P. Katsoulidis et al. Nature 565, 213 (2019) doi:10.1038/s41586-018-0820-9
Collaborators:
Alexandros P. Katsoulidis, Dmytro Antypov, George F. S. Whitehead, Elliot J. Carrington, Dave J. Adams, Neil G. Berry, George R. Darling, Matthew S. Dyer and Matthew J. Rosseinsky (Chemistry Department, The University of Liverpool).
Funding:
£3,359,331, European Commission, 1st Oct. 2016
£921,510, EPSRC, 1st Jul. 2012 – 1st March 2016